For people living with celiac, it is an unlabeled additive in food that may be as bad as gluten. For people without celiac, it may be partly responsible for the alarming rise in autoimmune disease. Microbial transglutaminase, or mTG, has been increasingly added to food over the past 30 years. It is known unappetizingly as “meat glue” because it gives hot dogs their unique texture, but it’s also used in many other foods. Read on to discover how this substance, which is FDA classifies as “Generally Recognized As Safe”, might have unrecognized side effects.
What Is mTG?
Transglutaminase is an enzyme found in many living things, and mTG is one type of this enzyme. It’s best known for creating a specific bond between amino acids in proteins. This bonding ability has led people to call it “meat glue.” Companies use mTG to improve the look, feel, and shelf-life of foods like meat, milk, and fish. It is even added to baked goods and sweets. mTG is also in other industries like beverages and oil, and even in health supplements.
One big reason companies use mTG is that it makes food last longer on store shelves. It’s a hot topic for food manufacturing, with many new patents being filed and mTG use expected to grow in the coming years. As for how much is used, up to 15 mg of mTG can be consumed in a day, and around 50-100 mg is needed to improve one kilogram of food.
Transglutaminase in the human gut comes from different places. Some of it is from the food we eat, like processed meat, cheese, yogurt, turkey roasts, and even in gluten-free baked goods. Other sources include probiotic supplements because mTG helps probiotic supplements survive the journey through the stomach to the lower intestines.
Our gut also has its own natural sources of the enzyme, coming from both good and bad bacteria, as well as yeasts and fungi. Finally, the cells lining our gut can also make transglutaminase. All these different sources mix together in the gut to modify proteins.
How Celiac Gets Started
In the initial development of celiac disease, it’s the gliadin portion of gluten that triggers the initial immune reaction. The enzyme tTG (tissue transglutaminase) modifies this gluten, making it more immunoreactive. After celiac disease is established, the body not only reacts to gluten but also to tTG. When someone with celiac disease eats gluten, the tTG enzyme modifies the gluten protein, triggering an immune response. The immune system then mistakenly targets not only the modified gluten but also the tTG enzyme itself, which is a natural part of the body. This is why antibodies against tTG can be used for diagnosing celiac disease. So, even though the initial reaction is to gluten, tTG’s interaction with gluten amplifies the immune response.
Why mTG Matters In Celiac
In celiac disease, the immune system mistakenly targets tissue transglutaminase (tTG). The tTG enzyme changes the structure of gluten, a protein found in wheat, rye, and barley. The modified gluten then becomes a target for the immune system, leading to inflammation and damage in the small intestine. This damage is what causes the symptoms and complications associated with celiac disease.
A big concern is the similarity of how mTG and tTG interact with gliadin, a component of gluten. Both tTG and mTG enzymes modify proteins, including gliadins.
They both create new complexes that trigger the immune system. This leads to the production of tTG antibodies that mark the beginning of celiac disease. When mTG treats wheat, it causes several immune responses.
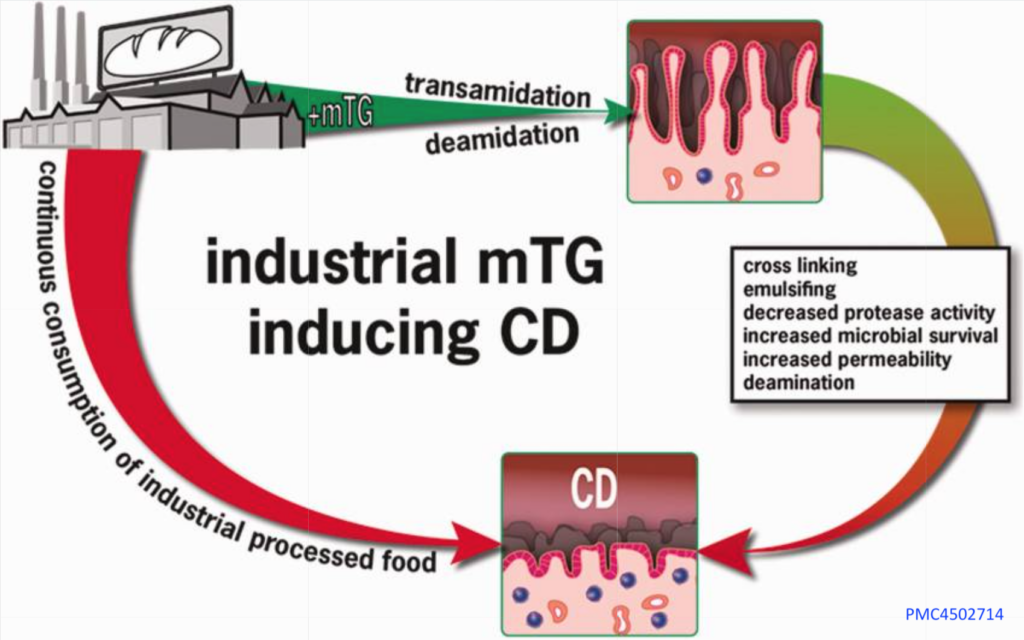
The mTG enzyme acts like a copycat of the tTG enzyme that modifies gluten in a way that triggers celiac disease. This tTG (tissue transglutaminase) is the actual target of the immune attack in people with celiac disease. [PMC4502714]
Researchers believe that mTG and other common food additives increase intestinal permeability. Leaky gut results in the entry of foreign immunogenic antigens that activate the autoimmune cascade. [PMID: 25676324]
Anti-mTG… antibodies correlate with intestinal damage to a comparable degree as anti-tTg.
Autoimmunity Reviews: The industrial food additive, microbial transglutaminase, mimics tissue transglutaminase and is immunogenic in celiac disease patients [PMID: 27640315]
How Microbial Transglutaminase Cross-Linked Complexes Might Cause Disease:
For people who do not have celiac, there is concern that mTG may lead to the development of the disease. [PMC8537092] Researchers cite the following reasons:
Immune Activation: Scientists used gold tagging to track gliadin and mTG through cells using electron microscopy. Both molecules move through cell compartments to the basolateral membrane. This location suggests these molecules interact with immune system cells to produce antibodies. The bonds formed by mTG and gliadin are stable and resistant to breaking down in the gut. This makes it even more likely that they’ll challenge the immune system.
Leaky Gut: Leaky gut might let things like bacteria, toxins, and even gliadin or gluten into the bloodstream. This can have an effect on the brain, possibly contributing to brain diseases and other conditions, including celiac disease. Additives in processed foods, along with certain enzymes, might also play a role in chronic diseases.
Increases Gliadin Uptake: The movement of gliadin peptides across the lining of the gut is helped by specific substances like secretory IgA and a receptor called apical transferrin receptor, especially when tTG (tissue transglutaminase) is present. Research shows that adding tTG to cells in a lab setting increases the uptake of gliadins. Since microbial transglutaminase (mTG) works similarly to tTG, it’s reasonable to think that mTG could also help gliadins enter the lining of the gut, which could make celiac disease worse. However, it’s not clear how mTG affects the barrier between the blood and the brain.
Suppression Protective Mucous Barriers: A healthy mucus layer in the gut helps keep harmful substances and bad bacteria away from the cells lining the gut. One key ingredient of this mucus is MUC2 mucin, which is a good target for the enzyme tTG (tissue transglutaminase). tTG modifies this mucin in a way that actually boosts its protective qualities. However, microbial transglutaminase (mTG) can mess with this mucin’s stability and make it easier for harmful substances to attach to gut cells. On the immune side of things, mTG from a specific bacteria, Streptococcus suis, hampers the ability of the immune system to engulf and remove harmful particles, weakening a key defense mechanism.
Contributes to Imbalances in The Gut Microbiome: Microbial transglutaminase (mTG) serves as a survival tool for microbes and also weakens gut immunity. For example, when scientists put the gene for mTG from one type of bacteria into another, Lactococcus lactis, the bacteria grew a lot more. Advances in bioengineering have made it easier and cheaper to produce this enzyme. The passage raises the question of whether bacteria that produce a lot of mTG could transfer this ability to other bacteria in the human gut, which could throw off the balance of the gut environment.
Potential Immune Activation: Certain immune cells in the intestine, like dendritic cells and macrophages, can interact with tissue transglutaminase (tTG). These cells can actually take in the enzyme. These immune cells might provide a new way for mTG and gliadins to come into contact with deeper immune cells in the tissue.
mTG Is Active Inside The Gut: Microbial transglutaminase, or mTG, forms complexes with gliadin that are resistant to the stomach’s digestive enzymes. mTG retains its activity up to 172°F (60°C) and remains active in environments with a pH of 4.0 or more. This can be significant because many individuals have reduced stomach acidity, either from eating, which temporarily neutralizes stomach acid, or due to the use of acid-suppressing medications. As a result, mTG might be active in various parts of the digestive system, forming complexes with gliadin that are tough and can stimulate an immune response.
To Dive Deeper
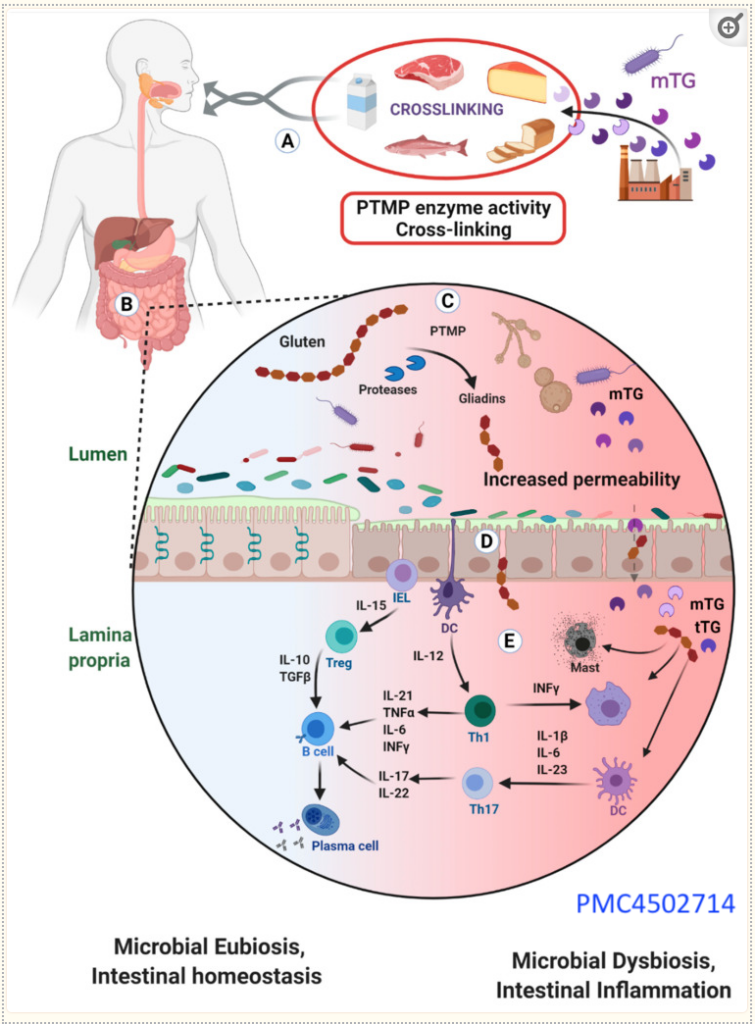
For those who are interested in the technical details, here’s more of a step-by-step breakdown of how mTG from processed foods affects your gut and immune system:
(A) When you eat foods like meat, fish, dairy, and bread that have been treated with mTG, this additive enters your digestive system.
(B) Inside your gut, mTG sticks to certain food particles, forming complexes.
(C) Proteins found in wheat, called gliadins, are easily bound by mTG, which can change them in a way that prompts an immune response. Not only that, but mTG can also come from other foods and microbes like yeast, leading to more binding and possible gut irritation.
(D) mTG might disrupt the protective mucus lining inside the gut, weakening the barriers that keep harmful substances out of your gut wall. If gluten is present, it can make this barrier even leakier, letting these complexes pass deeper into the gut lining.
(E) Once inside the deeper layers of the gut, these complexes can fire up the immune system, leading to inflammation. This involves various immune cells, like those that release signals to call more immune cells to the area, potentially causing more inflammation.
mTG Usage Should Be Labeled and Declared on Food Products
There are growing concerns about the use of microbial transglutaminase (mTG) in the food industry. Regulatory agencies, academic experts, and social media influencers are all issuing warnings. One key point is the call for transparency, requiring food products to declare the use of transglutaminase on their labels. This is particularly critical for those with celiac disease, as mTG can increase the immunogenicity of gluten. In countries like Switzerland, Germany, and Canada, the public has been notified about these safety concerns, and the enzyme must be labeled on the final product.
To Sum It Up
Microbial transglutaminase, a kind of “food glue,” has been used in food processing for about thirty years and its use is still growing. Although this enzyme is recognized as generally safe and is used to bind proteins together, it has some negative aspects that are often overlooked. It doesn’t have to be listed on food labels because it’s considered a processing aid, not an ingredient, which means it isn’t subject to strict safety testing. This enzyme can weaken the gut’s protective barriers and increase the risk of immune reactions and inflammation. It acts like some of our body’s own enzymes and might play a role in various long-term health problems. Authorities are being called to reevaluate how microbial transglutaminase is classified and to consider labeling it on food products and checking its safety more closely. Changes in these regulations could significantly alter food labeling and safety checks.

 Scan Me!
Scan Me!